Abstract
Background: Follicular lymphoma (FL) is a common subtype of indolent lymphomas, and accounts for about 10% of all non-Hodgkin lymphoma(NHL) cases. FL patients generally respond well to the 1 st line therapy, but most patients would then relapse, and duration of response gradually decreases as treatment line escalates. For FL patients of 3 rd line and beyond, treatment options are limited, and recurrent chemotherapy also greatly impact their quality of life. Four PI3Kδ inhibitors have been approved in the US for adult patients with relapsed or refractory FL. Currently, multiple trials investigating PI3K inhibitors in R/R FL are on-going in China, yet none has been approved. Parsaclisib, a potent, highly-selective, next-generation PI3Kδ inhibitor, has shown promising efficacy and tolerance in patients with previously treated B-cell malignancies. Here, we report interim result of CIBI376A201 (NCT04298879), a multicenter, open-label phase 2 study of parsaclisib in 3 rd line FL patients in China.
Methods: Key eligibility included, age ≥18 years, histologically confirmed FL grade 1, 2, or 3a, ≥2 prior systemic therapies, Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2, and ineligible for hematopoietic stem cell transplantation (HSCT). Patients were allocated to receive parsaclisib 20 mg once daily (QD) for 8 weeks followed by 2.5 mg QD, till disease progression, intolerable AE or withdrawal from study due to other reasons. Prophylaxis for Pneumocystis jirovecii pneumonia (PJP) was required. Primary end point was objective response rate (ORR) evaluated by independent review committee (IRC), secondary end points included: ORR by investigator, duration of response (DOR), progression-free survival (PFS), overall survival (OS), pharmacokinetic characteristics and safety and tolerability.
Results: From April, 2020 to 11 th, April 2021 (data cut-off), 36 patients were treated. Enrollment is ongoing. At cut-off date, 5 (13.9%) patients had discontinued treatment due to PD (progressive disease)/PMD (progressive metabolic disease). The median exposure (range) was 104 days (5-354 days). The median age was 51 years, and 52.8% of the patients were male. The median time since initial diagnosis was 3.3 years. At enrollment, most patients (88.9%) had an ECOG PS ≤1, and 55.6% had a Follicular Lymphoma International Prognostic Index score ≥3 (high risk). All patients had ≥2 lines of prior systemic therapy, in which 22.2% had 3 or more prior lines.
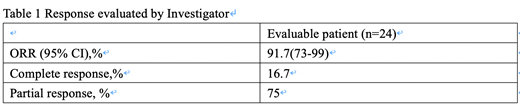
At the data cut-off date, 24 patients were evaluable for response. According to the investigator's evaluation, the ORR and CRR were 91.7% (95% confidence interval [CI]: 73%−99%) and 16.7% (95% confidence interval [CI]: 4.7%-37.4%) respectively, in all evaluable patients. The median DOR was not reached among responders overall.
Among the 36 patients evaluable for safety, the most common treatment-emergent adverse events (TEAE) were neutrophil count decrease (36.1%), white blood cell count decrease (16.7%), platelet count decrease (16.7%), anemia (13.9%), upper respiratory tract infection (11.1%), ALT elevation (11.1%) and diarrhea (11.1%). The most common grade ≥3 TEAEs were neutrophil count decrease (8.3%), platelet count decrease (2.8%), upper respiratory tract infection (2.8%) and anemia (2.8%). 22.2% of patients had dose interruption due to TEAE, no dose reduction occurred due to TEAE. No treatment discontinuation occurred due to TEAE. Serious TEAEs included upper respiratory tract infection (2.8%), organ dysfunction (2.8%), dizziness (2.8%) and nasal cavity mass (2.8%), all unrelated to the study drug. One patient (2.2%) died during the trial, considered unrelated to studied drug by investigator.
Conclusion: Parsaclisib demonstrated promising efficacy, and was generally well tolerated. These results demonstrate parsaclisib could bring substantial benefit for 3 rd line FL patient. Updated data will be presented in the future.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal